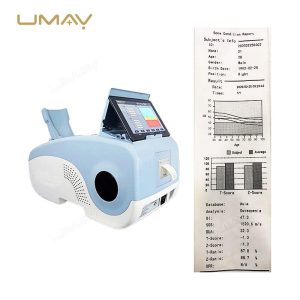







High-Quality Calcaneus Ultrasound Bone Densitometer for Bone Health Assessment
SKU: UMY-US-034
Brand: Umy
MOQ: 1
Origin: China
Quick Info.
- SKU NO.: UMY-US-034
- Device Classification: Class Ⅱ
- Warranty: 1 Year
- Power Source: Electric
- Transport Package: Carton
- Origin: China
- Material: Metal, Plastic
- After-Sale Service: Online Technical Support
- Production Capacity: 6000 Sets/Year
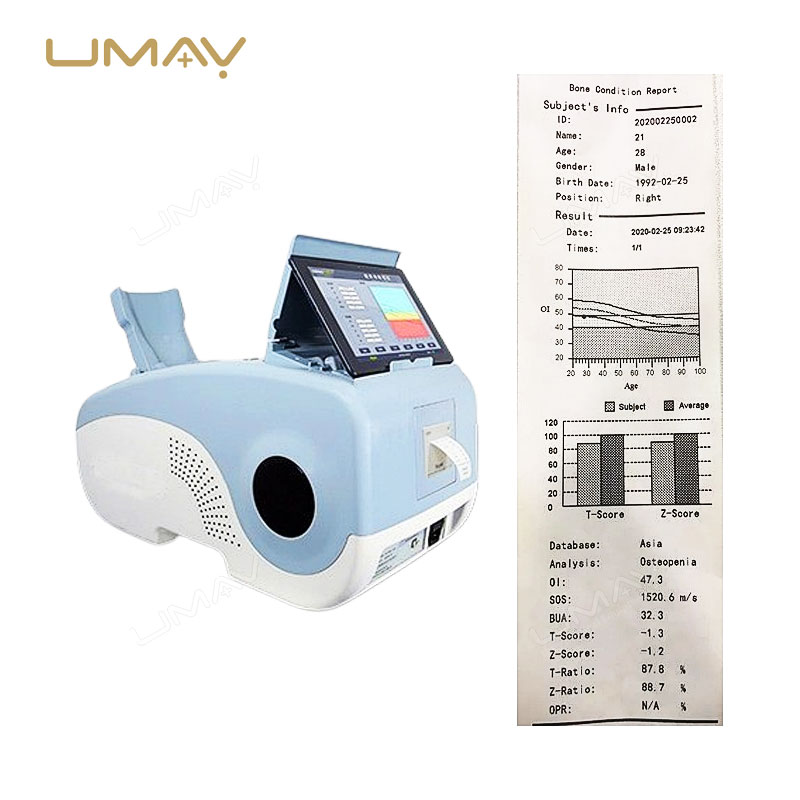
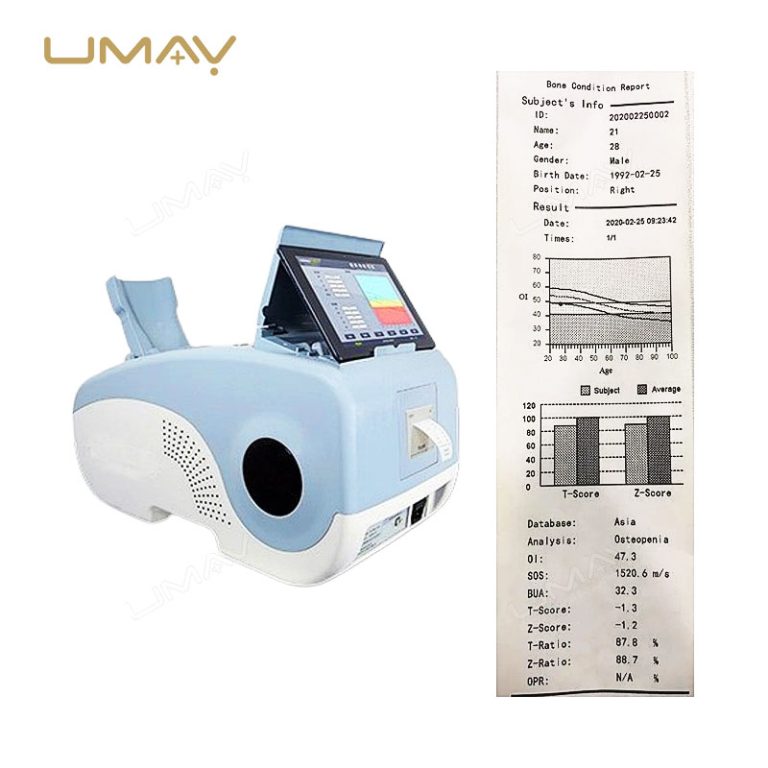
This high-quality calcaneus ultrasound bone densitometer is designed for precise bone density measurement in the heel, aiding in early detection of osteoporosis and other bone health issues. Utilizing advanced ultrasound technology, it provides accurate, non-invasive results without radiation exposure, making it safe for frequent use. Compact and efficient, it is suitable for clinics, hospitals, and mobile healthcare settings. This densitometer is essential for healthcare providers focused on comprehensive bone health monitoring.
The Specific Parameters
| ITEMS | PARAMETERS |
|---|---|
| Ultrasonic Parameters | |
| BUA | Ultrasonic amplitude attenuation |
| SOS | Ultrasonic sound velocity |
| OI | Bone mass index |
| Measurement Method | |
| Type | Full dry type (no liquid inside and outside the equipment, no temperature control required) |
| Transmission | Two-way ultrasonic transmission and reception |
| Probe Specifications | |
| Frequency | 0.5MHz ± 10% |
| Broadband (at 4-6dB) | >60% |
| Measurement Details | |
| Measurement Time | ≤ 25 seconds |
| Test Repeatability (OPR) | ≤ ±1% |
| Measurement Accuracy (SOS) | ≤ ±2% |
| Test Repeatability (BUA) | ≤ ±5% |
| Diagnostic Parameters | |
| Parameters | BUA, OI value, T value, Z value, SOS, OPR, adult ratio, age ratio, obesity index, height prediction, BMI, child Z value chart |
| Ultrasonic Output | |
| TIS | 2.8 × 10^-3 mW/cm² |
| Calibration | |
| Correction | Automatic calibration of the human body simulation module |
| Temperature Compensation | |
| System | Automatically compensates for measurement deviation caused by temperature |
| Reference Database | |
| Regions | Asian, European, Middle Eastern, and African databases |
| Interface and Output | |
| Interface | Dual USB, external tablet, built-in thermal printer, external printer support |
| Report Types | Diagnostic report, PACS network system-compatible |
| Measurement Site | |
| Probe Spacing | Automatically adjusts for direct contact with the heel |
| Bone Density Software | |
| System | Child and adult bone density test software, automatic probe location and best signal search |
| Prompts | Automatic test site placement prompts |
| Parameters | |
| Adults | T value, Z value, age ratio, adult ratio, OPR, OI, SOS, BUA |
| Children | Z value, BMI, height prediction, obesity, SOS, BUA |
| Additional Features | |
| Animation Playback | Attracts children’s attention during examination |
| Probe | Permanent oil bladder probe, no replacement required |
| Interface and Reports | |
| Language Switching | Chinese/English interface |
| Report Formats | A4, B5, and others, preview and print options |
| Bluetooth | Supports Bluetooth report transmission |
| Supports WeChat scan code self-download | |
| Networking | |
| Internet Functions | Supports hospital network systems and expert remote consultation |
| Data Management | |
| Case Database | Automatic recording, query, classification, backup; export results to Excel |
| Environmental Requirements | |
| Operating Humidity | 30-70% (non-condensing) |
| Operating Temperature | 10-40°C |
| Power Requirements | |
| Voltage | AC220V ± 10% |
| Frequency | 50Hz |
| Power | 3.15A, 125W |
| Instrument Specifications | |
| Weight | 13kg |
| Size (W×H×L) | 330mm × 360mm × 645mm |